생리식염 주사액(KP)
1) 시험기준
(1) 0.85 ~ 0.95 w/v%
2) 시험방법
(1) 이 약 20 mL를 정확하게 취하여 물 30 mL를 넣는다.
(2) 지시약 플루오레세인나트륨시액 3방울 넣는다.
(3) 세게 흔들어 섞으면서 0.1 mol/L 질산은액으로 적정한다.
0.1 mol/L 질산은액 1 mL = 5.844 mg NaCl
<계산식>
| 정량(w/v%) | = | A × F × 5.844 | x 100 |
| 20 mL × 1000 |
• 0.1 mol/L 질산은액 1 mL = 5.844 mg NaCl
• A : 본시험 시 0.1 mol/L 질산은액의 소비량(mL)
• F : 0.1 mol/L 질산은액의 규정도계수
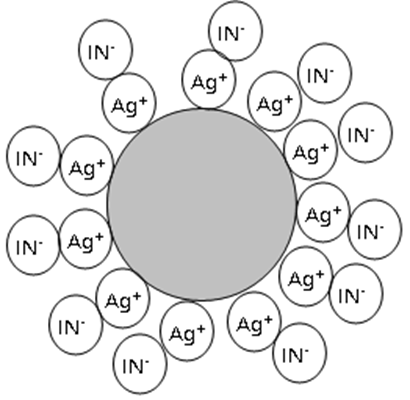
Fajans method of chloride determination employs an adsorption indicator. The indicator reaction takes place at the surface of the precipitate. The indicator is a weakly acidic dye and exists in solution in the ionized form, In-. The titrant is a silver solution, and during the titration a precipitate of AgCl is formed. Initially this precipitate is colloidal, consisting of very small non-settling particles with a diameter of less than 1 µm. While this would be undesirable for a gravimetric determination (colloids cannot be filtered), it is advantageous for an adsorption indicator method. What happens is the following:
Precipitates have a tendency to adsorb “their own” ions to the surface to form what is known as the primary adsorption layer, i.e., AgCl preferentially adsorbs Ag+ or Cl-, whichever happens to be in excess. A colloidal precipitate has a very large surface area and, therefore, presents an abundance of room for adsorption. Before the equivalence point of the titration of Cl- with Ag+, the Cl- ion is in excess and forms the primary adsorption layer on the surface of the AgCl precipitate. The particles have a negative surface charge and repel each other; the colloid is stabilized by this. The indicator ion, In‑, is also repelled and stays well away from the surface. Because the particles are negatively charged, they attract cations that are in solution more strongly than anions. Thus there is weakly bound secondary adsorption layer consisting of the cation that forms the most insoluble chloride to AgCl (probably Na+); these ions form the secondary adsorption layer.
Beyond the equivalence point, Ag+ is in excess and the surface of the precipitate becomes positively charged, with the primary layer being Ag+. These positively charged colloidal particles will now attract the indicator anion and adsorb it into secondary adsorption layer.
The indicator forms a colored complex with silver ion, imparting a color to the precipitate. Only at the surface is the silver ion concentration high enough for the solubility product of the complex to be exceeded; this does not happen anywhere else in the solution, and the color is therefore confined to the precipitate surface.
The pH must be controlled for reliable results. If it is too low, the indicator (a weak acid) is dissociated too little to produce enough In-. In the case of Fajans method, dichlorofluorescein is the preferred indicator and it gives good results at pH values around 7.
Since the end point does not exactly coincide with the equivalence point, the titrant should the standardized by the same titration as used for the sample (this eliminates the inherent error). Photodecomposition of AgCl, creating a purple-black hue on top of the white AgCl, is another source of error. It should be minimized by carrying out the titration expeditiously and in relative low light. As explained above, a colloidal precipitate is preferred in this titration. At the equivalence point, neither titrant nor titrate ions are in excess, and the precipitate is momentarily without a surface charge. This causes the colloidal particles to coagulate, thereby reducing the precipitate surface area. It can be prevented by the addition of a small amount of dextrin (hydrolyzed starch) to the solution.
The titration reaction is:
AgNO3 (aq) + NaCl (aq) ® AgCl (s) + NaNO3 (aq)
In fact, because neither the Na+ nor NO3- ions are involved in the reaction, this reaction may be written more accurately as:
Ag+ + Cl- º AgCl(s)
The nitrate ions are only weakly adsorbed to the precipitate after the equivalence point is reached, and they are easily displaced by indicator ion. The end point is signaled by the appearance of the pink color of silver dichlorofluoresceinate.
'품질관리(Quality Control) > 이화학' 카테고리의 다른 글
| JP INFRARED REFERENCE SPECTRA IR 스펙트럼 참조 (0) | 2025.04.22 |
|---|---|
| 제약회사 GMP 성적서 CoA 관련 글 (0) | 2025.04.18 |
| GMP 제약회사 품질관리 유효숫자 및 반올림 방법 (0) | 2025.04.04 |
| 용량분석용표준액 0.1 mol/L 과염소산 표정 방법 (1) | 2025.02.27 |
| 이산화황 시험법(KP) 대한민국약전 일반시험법 이산화황시험 이 글로 종결!! (1) | 2025.02.20 |
