저울을 2대 구입해서 SOP 작성하려고하니 참고할만한 자료를 구하다 보니 자연스럽게 공부하게 되었다. 제약회사 품질관리 부서에서 근무하게되면 기준서나 SOP에 따라 진행하면 된다.
저울만해도 이렇게 공부할게 많다니.. 오늘은 저울 점검 중 반복성에 대해 작성하려한다. 일단 검색만하면 관련 내용이 많지만, 좀 더 세세히 이해되도록 작성하려 한다. 영어를 한글로 바꾸면 쫌.. 이상해져서 그냥 영어 위주로 올린다.
USP 내용
In the pharmaceutical industry, the general chapters (GC) <41> and <1251> of the United States Pharmacopoeia (USP) are widely recognized as standards for managing balances, and how a minimum weight is calculated is also stipulated in these chapters.
Repeatability requirements
1) Repeatability is assessed by measuring one test weight not fewer than 10 times.
2) Repeatability is satisfactory when two times the standard deviation (S.D.) of the weighed values, divided by the desired smallest net weight (the smallest sample quantity that users actually intend to measure with the balance), does not exceed 0.10%.
These conditions can be expressed as:
(2 × S.D. of not fewer than 10 repeated weighing) / smallest net weight ≤ 0.10%
In other words, the following can be said: Minimum weight ≥ 2000 × Repeatability (S.D.)
[NOTE—The test weight must be within the balance’s operating range, but the weight need not be calibrated. Because the standard deviation is virtually independent of sample mass within the balance’s capacity, use of a small test weight, which may be difficult to handle, is not required.]
The minimum weight, Mmin, is described by the inequality Mmin ≥2000s.
For example, if s is found to be 0.00015, then Mmin must be ≥ 0.30000 g or 300.00 mg. If the standard deviation, s, obtained is less than 0.41d, where d is the scale interval of the balance, then the inequality becomes Mmin ≥ 2000 (0.41d). For example, for a 4-place analytical balance, d is 0.0001 so that Mmin must be ≥ 0.0820 g or 82 mg.
왜 이렇게 되는지... 처음보고 100% 이해가 되지 않는다. 인터넷에 올라와있는 자료를 봐도.. 노이해.. 어떤 글은 분동값을 넣으라는 글도 봤다.....
엥~~~ 0.41d 이건 또 모야......................... 그래서 찾아봤다.....
With the rectangular distribution, an estimate of the standard uncertainty related to the indication rounding, can be calculated.


Formula for the minimum weight:
mmin = 2 × s / Required weighing tolerance
For a tolerance of 0.10%:
mmin = 2 × s / 0.10%
mmin = 2 × s × 1000
mmin = 2000 × s
When s = 0.41d:
mmin = 2000 × 0.41d
mmin = 820 × d
반복성 점검은 감도 점검에 비해 적은 빈도로 수행되지만, 반복성은 낮은 칭량 범위의 경우 측정불확도에 가장 많이 기여하는 요인이므로 작은 샘플을 칭량할 경우 반복성 점검이 매우 중요하다.
반복성은 같은 측정 조건에서 하나의 동일한 하중을 반복적으로 칭량할 때 같은 결과를 제공할 수 있는 저울의 능력을 측정한다. 반복성은 보통 동일한 시험 분동을 사용하여 10회 칭량을 반복 수행하는 방법으로 측정한다.
시험 절차
1. Tare the balance
2. Place the test weight on the centre of the weighing pan, print or note the value.
3. Lift the test weight from the pan, press tare.
4. Repeat Steps 2 and 3 for the next 9 weighing steps.
5. Calculate the standard deviation.
Repeatability and minimum sample weight
The requirement for repeatability restricts the weighing range to a working range usable for the corresponding purposes in the form of a minimum sample weight. The minimum sample weight is calculated from the standard deviation s of a repeatability measurement with at least 10 repetitions according to:
| Mmin = | 2000 * s | If s ≥ 0.41 * d |
| 2000 * 0.41 * d | otherwise |
The minimum sample weight according to USP is therefore determined pragmatically so that any sample weights are larger than random deviations by a factor of 2000 (or than its standard deviation, to be mathematically correct). The second line in the formula above only limits this to a minimum value of 2000 · 0.41 · d = 820 · d, as otherwise a measured standard deviation of s = 0 g would result in a minimum sample weight of mmin,USP = 0 g.
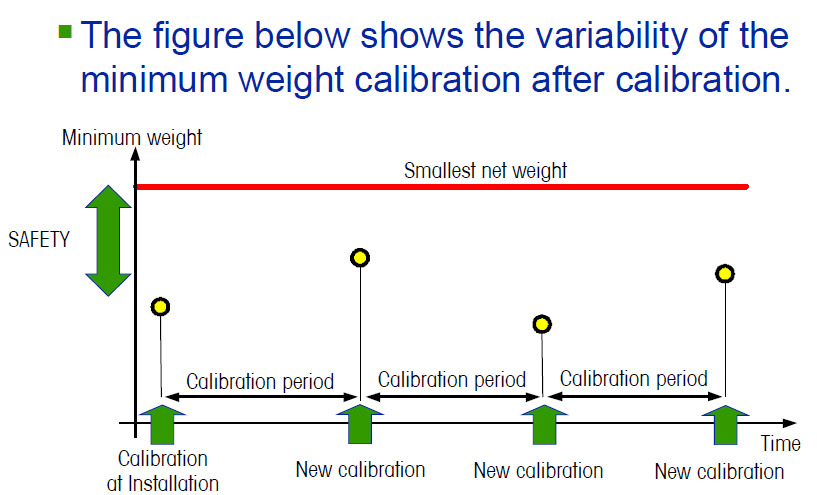
The minimum sample weight according to USP, chapter <41>, therefore not only takes the characteristics of the balance in the form of the scale interval d, but also the actual condition or ambient conditions in the form of the standard deviation of the repeatability measurement.
The minimum sample weight can therefore reasonably be determined only at the balance's site of use and should be repeated if there is a change to the installation location. Even under ideal conditions, the minimum sample weight according to chapter <41> USP is always at least 820 · d – this value must never be fallen short of.
The overview "Minimum and typical values for minimum sample weights according to USP <41>, OIML R 76 and EURAMET cg-18" lists realistically achievable values for common Sartorius balance models – provided the installation conditions are good.
Remark: It is generally not recommended to repeatedly adapt the current working range to the minimum sample weight value resulting from the last repeatability measurement – since the standard deviation is a statistical
variable, it can always vary slightly from measurement to measurement. So if users get obtain a standard deviation of, for example, if users get the standard deviation of, for example, s = 5 μg from a repeatability measurement, set the minimum sample weight for their balance accordingly to 2000 · s = 10 mg, and then weigh samples down to this value, they risk that at the next repeatability measurement s = 8 μg is obtained, and therefore a minimum sample weight of 2000 · s = 16 mg results. In this case, it would now be questionable whether past sample weights between 10 mg and 16 mg met the USP requirements. We therefore recommend defining a "desired minimum sample weight" for each balance, which is reliably adhered to – checking the balance according to USP then ensures that the actual minimum sample weight is smaller than the defined one. The permissible minimum sample weight should be visibly marked on balances that are used under USP provisions. Some Sartorius balances permit the minimum sample weight to be specified in the service menu. Net sample weights below the minimum sample weight can therefore not be made (see section regarding the SQmin function).
반복성 계산 예시
복잡하게 설명하는것보다 예시로 보면 쉽게 이해할 수있다. 생각보다 타이트하다...
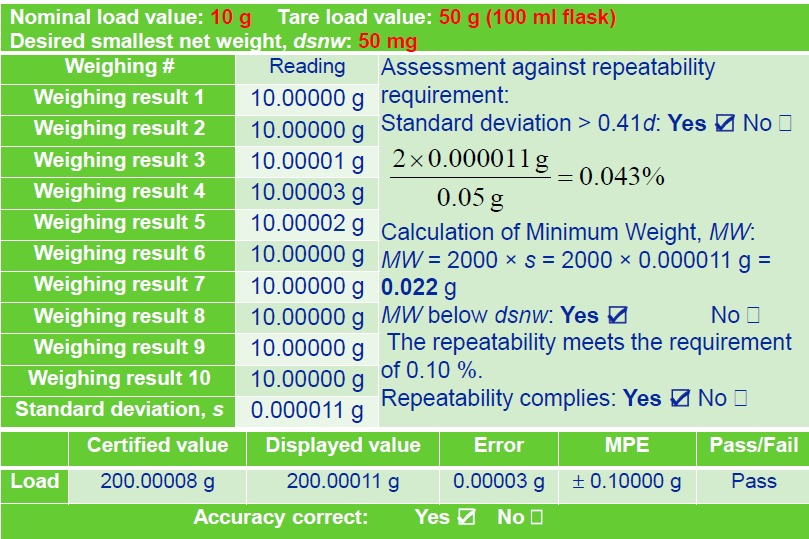
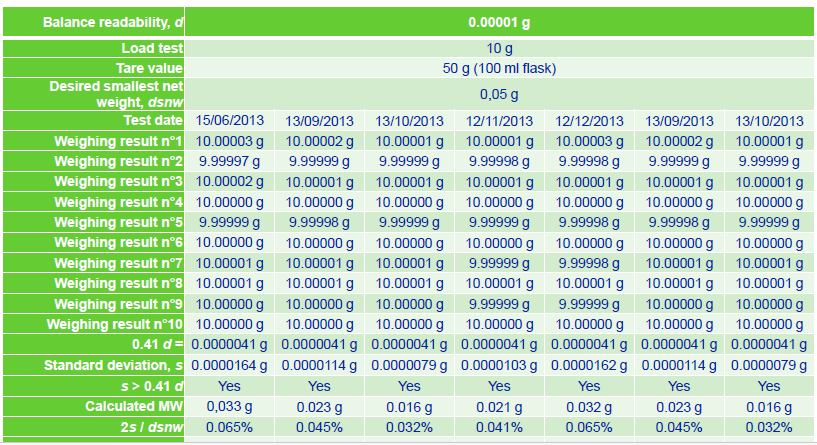

여기 까지 보면 이해했겠지............... 다음편은 직선성 정밀도 편심오차 등등~~ 저울도 내용이 많네.... ㅋㅋ
점검 엑셀 파일도 작성중에 있다.. 다음엔 엑셀 파일도 올려야지~~ (계획한 엑셀 파일은.. 내가 사용 목적으로 제작 중...
GMP 회사에서 말고~~컴퓨터 연결 자동 입력 및 계산)
GMP.... ㅜ
'품질관리(Quality Control) > 일반업무' 카테고리의 다른 글
| 제약회사 품질관리팀 신입사원 실제 업무 일과 (13) | 2025.07.22 |
|---|---|
| 제약회사 품질관리 신입사원이면 무조건 하는 업무 : 기기 점검(저울, pH 등) 방법 (23) | 2025.07.07 |
| 제약회사 품질관리팀에서는 무슨 일을 할까? 다른 직업 추천해요~ (9) | 2025.07.06 |
| GMP 제약회사 품질관리 QC 신입사원 교육 프로그램 SOP (0) | 2025.04.04 |
| 제약회사 표준품 사용 관리 (1) | 2025.01.29 |
